⭐NEW⭐ Ester Expósito Age 2026 Vault HQ Vids/Pics Full Link
Browse the private ester expósito age exclusive feed freshly updated today. Our platform provides a massive collection of high-definition videos, private photos, and unreleased files. For your convenience, we provide one-click media downloads with no subscription fees. Enjoy ester expósito age in stunning 4K clarity. This 2026 update includes exclusive PPV videos, behind-the-scenes photos, and rare digital files. Get the freshest ester expósito age video uploads. Click the download link now to view the entire collection.
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl group (−oh) of that acid is replaced by an organyl group (r ′) In chemistry, an ester is a compound derived from an acid (organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl group (−oh) of that acid is replaced by an organyl group (−r). [1] these compounds contain a distinctive functional group.
Ester - Definition, Structure, Esterification along with Properties & Uses
Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids You will also learn about esterification and its mechanism. Esters derived from carboxylic acids are the most common
Learn about the different types and reactions of esters and more in this article.
The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups Esters are derived from the condensation reaction between a carboxylic acid and an alcohol, resulting in the elimination of water. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group Esters are derived from carboxylic acids and (usually) alcohol.
Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. The meaning of ester is any of a class of often fragrant organic compounds that can be represented by the formula rcoor' and that are usually formed by the reaction between an acid and an alcohol with elimination of water. The ester linkage is also present in animal fats and in many biologically important molecules The chemical industry uses esters for a variety of purposes
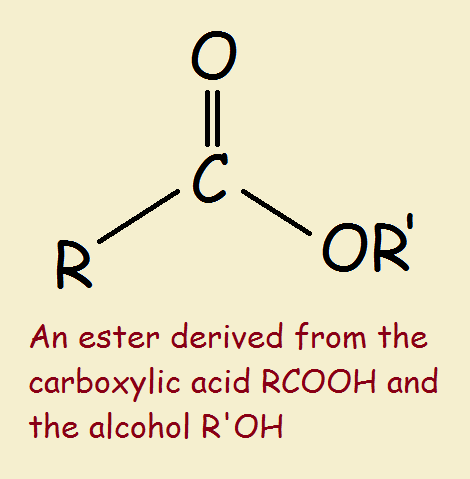
Ethyl acetate, for instance, is a commonly used solvent, and dialkyl phthalates are used as plasticizers to keep polymers from becoming brittle.
In this tutorial you will learn about the basic properties and structure of an ester functional group